De Novo Synthesis of Purine Nucleotides
Synthesis of Purine Nucleotides
In Synthesis of Purine Nucleotides
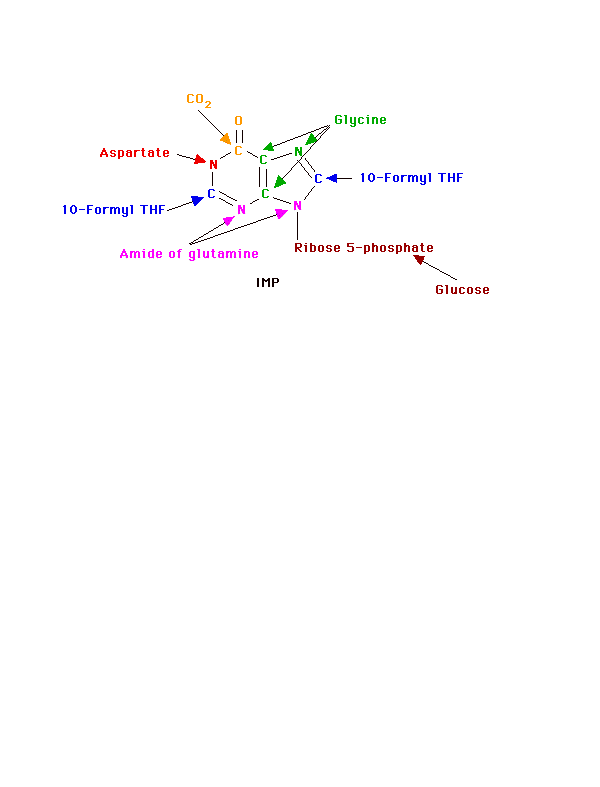
We use for purine nucleotides the entire glycine molecule (atoms 4, 5,7), the amino nitrogen of aspartate (atom 1), amide nitrogen of glutamine (atoms 3, 9), components of the folate-one-carbon pool(atoms 2, 8), carbon dioxide, ribose 5-P from glucose and a great deal of energy in the form of ATP. In de novo synthesis, IMP is the first nucleotide formed. It is then converted to either AMP or GMP.
Synthesis of Purine Nucleotides
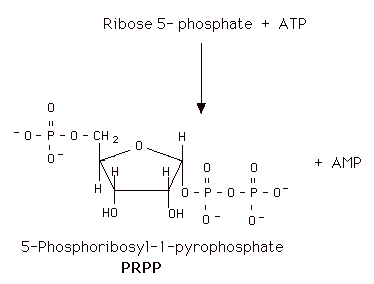
PRPP
Since the purines are synthesized as the ribonucleotides, (not as the free bases) a necessary prerequisite is the synthesis of the activated form of ribose 5-phosphate. Ribose 5-phosphate reacts with ATP to form 5-Phosphoribosyl-1-pyrophosphate (PRPP).
Synthesis of Purine Nucleotides
This reaction occurs in many tissues because PRPP has a number of roles - purine and pyrimidine nucleotide synthesis, salvage pathways, NAD and NADP formation. The enzyme is heavily controlled by a variety of compounds (di- and tri-phosphates, 2,3-DPG), presumably to try to match the synthesis of PRPP to a need for the products in which it ultimately appears.
Commitment Step
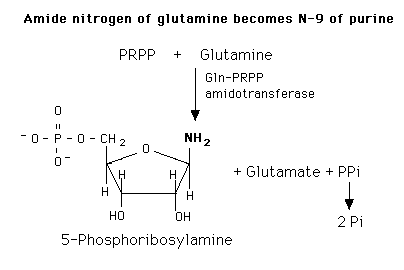
De novo purine nucleotide synthesis occurs actively in the cytosol of the liver where all of the necessary enzymes are present as a macro-molecular aggregate. The first step is a replacement of the pyrophosphate of PRPP by the amide group of glutamine. The product of this reaction is 5-Phosphoribosylamine. The amine group that has been placed on carbon 1 of the sugar becomes nitrogen 9 of the ultimate purine ring. This is the commitment and rate-limiting step of the pathway.
Synthesis of Purine Nucleotides
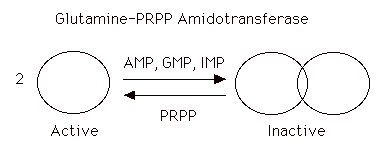
The enzyme is under tight allosteric control by feedback inhibition. Either AMP, GMP, or IMP alone will inhibit the amidotransferase while AMP + GMP or AMP + IMP together act synergistically. This is a fine control and probably the major factor in minute by minute regulation of the enzyme. The nucleotides inhibit the enzyme by causing the small active molecules to aggregate to larger inactive molecules.
[PRPP] also can play a role in regulating the rate. Normal intracellular concentrations of PRPP (which can and do fluctuate) are below the KM of the enzyme for PRPP so there is great potential for increasing the rate of the reaction by increasing the substrate concentration. The kinetics are sigmoidal. The enzyme is not particularly sensitive to changes in [Gln] (Kinetics are hyperbolic and [gln] approximates KM). Very high [PRPP] also overcomes the normal nucleotide feedback inhibition by causing the large, inactive aggregates to dissociate back to the small active molecules.
Purine de novo synthesis is a complex, energy-expensive pathway. It should be, and is, carefully controlled.
Formation of IMP
Once the commitment step has produced the 5-phosphoribosyl amine, the rest of the molecule is formed by a series of additions to make first the 5- and then the 6-membered ring. (Note: the numbers given to the atoms are those of the completed purine ring and names, etc. of the intermediate compounds are not given.) The whole glycine molecule, at the expense of ATP adds to the amino group to provide what will eventually be atoms 4, 5, and 7 of the purine ring (The amino group of 5-phosphoribosyl amine becomes nitrogen N of the purine ring.) One more atom is needed to complete the five-membered ring portion and that is supplied as 5, 10-Methenyl tetrahydrofolate.
Before ring closure occurs, however, the amide of glutamine adds to carbon 4 to start the six-membered ring portion (becomes nitrogen 3). This addition requires ATP. Another ATP is required to join carbon 8 and nitrogen 9 to form the five-membered ring.
The next step is the addition of carbon dioxide (as a carboxyl group) to form carbon 6 of the ring. The amine group of aspartate adds to the carboxyl group with a subsequent removal of fumarate. The amino group is now nitrogen 1 of the final ring. This process, which is typical for the use of the amino group of aspartate, requires ATP. The final atom of the purine ring, carbon 2, is supplied by 10-Formyl tetrahydrofolate. Ring closure produces the purine nucleotide, IMP.
Note that at least 4 ATPs are required in this part of the process. At no time do we have either a free base or a nucleotide.
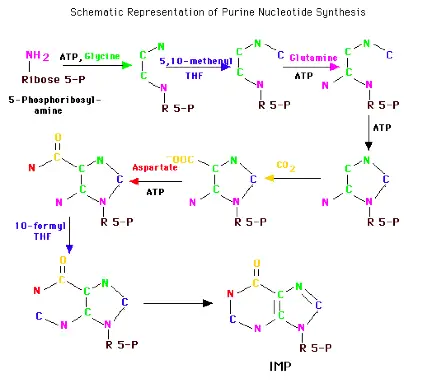
Formation of AMP and GMP
IMP can then become either AMP or GMP. GMP formation requires that IMP be first oxidized to XMP using NAD. The oxygen at position 2 is substituted by the amide N of glutamine at the expense of ATP. Similarly, GTP provides the energy to convert IMP to AMP. The amino group is provided by aspartate in a mechanism similar to that used in forming nitrogen 1 of the ring. Removal of the carbons of aspartate as fumarate leaves the nitrigen behind as the 6-amino group of the adenine ring. The monophosphates are readily converted to the di- and tri-phosphates.
Synthesis of Purine Nucleotides
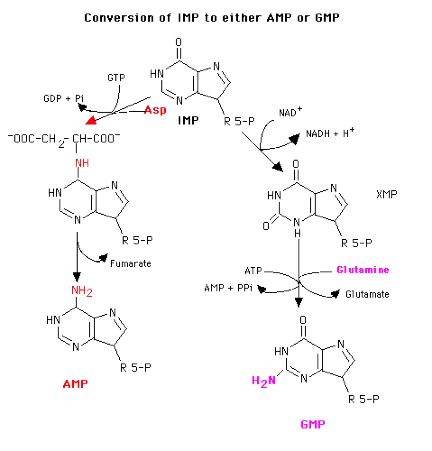
Control of De Novo Synthesis
Control of purine nucleotide synthesis has two phases. Control of the synthesis as a whole occurs at the amidotransferase step by nucleotide inhibition and/or [PRPP]. The second phase of control is involved with maintaining an appropriate balance (not equality) between ATP and GTP. Each one stimulates the synthesis of the other by providing the energy. Feedback inhibition also controls the branched portion as GMP inhibits the conversion of IMP to XMP and AMP inhibits the conversion of IMP to adenylosuccinate.
Synthesis of Purine Nucleotides
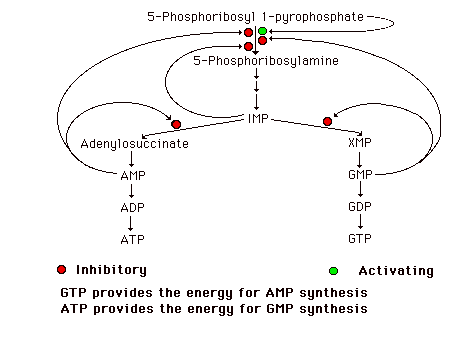
Possible Scenario:
One could imagine the controls operating in such a way that if only one of the two nucleotides were required, there would be a partial inhibition of de novo synthesis because of high levels of the other and the IMP synthesized would be directed toward the synthesis of the required nucleotide. If both nucleotides were present in adequate amounts, their synergistic effect on the amidotransferase would result in almost complete inhibition of de novo synthesis.
Synthesis of Purine Nucleotides
De Novo Synthesis of Pyrimidine Nucleotides
Since pyrimidine molecules are simpler than purines, so is their synthesis simpler but is still from readily available components. Glutamine's amide nitrogen and carbon dioxide provide atoms 2 and 3 or the pyrimidine ring. They do so, however, after first being converted to carbamoyl phosphate. The other four atoms of the ring are supplied by aspartate. As is true with purine nucleotides, the sugar phosphate portion of the molecule is supplied by PRPP.
Synthesis of Purine Nucleotides
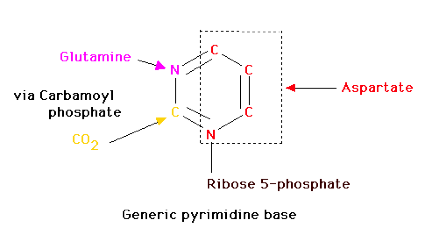
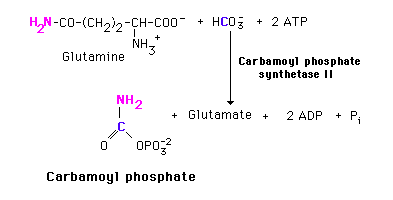
Carbamoyl Phosphate
Pyrimidine synthesis begins with carbamoyl phosphate synthesized in the cytosol of those tissues capable of making pyrimidines (highest in spleen, thymus, GItract and testes). This uses a different enzyme than the one involved in urea synthesis. Carbamoyl phosphate synthetase II (CPS II) prefers glutamine to free ammonia and has no requirement for N-Acetylglutamate.
Synthesis of Purine Nucleotides Reprint Permission Given to Gout-Aware All Articles written & supplied by
Dr Carol .N. Angsatdt Ph.D Department of Biomedical Sciences Allegheny University of Health Sciences

Recent Articles
-
Tony
May 08, 24 08:57 PM
Hi Peter, I am a physician, had my first Gout episode last week. I was surfing through the web and happened to come across your site. I am impressed by -
What is it about Cherries?
Aug 25, 21 03:59 AM
Allopurinol worked for me, but at the expense of adversely affecting my mood.I like cherries, but getting them every day of the year is a problem.I wondered -
ACV made my gout worse...
Apr 22, 20 07:19 AM
During my first gout attack, I was in so much pain that I desperately scoured the internet for alternative cures as the colchicine that I was prescribed







